Clinical research is the source of all medical progress. However, our current clinical trial system is slow, expensive, and imprecise. Funding the development of autonomous AI clinical researchers can fix this.
Problems Autonomous Clinical Research Could Solve
Clinical Research is Expensive
It costs $2.6 billion to bring a drug to market (including failed attempts). It costs $36k per subject in Phase III clinical trials.

We Don’t Know the Effects of Thousands of Chemicals in Our Food
Lots of these diseases are caused or worsened by chemicals in your food, but we don’t really know which ones, We only have long-term toxicology data on 2 of the over 7000 preservatives, flavorings, emulsifiers, sweeteners, pesticides, contaminants, and herbicides in your diet.

The increase in the number of chemicals has been linked to increases in the incidence of many diseases associated with disrupted gut microbiomes.
No Incentive to Discover Benefits of Off-Patent Treatments
Once a drug’s patent expires, there’s often little financial incentive to explore new uses for it, even if preliminary evidence suggests it could be effective for treating other conditions. This results in a significant loss of potential medical advancements. Off-patent drugs are already approved for safety, meaning they could be brought to market for new uses much more quickly and at a lower cost than new drugs, but the current funding model doesn’t support this kind of repurposing.
We Don’t Know the Long-Term Effects of Drugs
It’s not financially feasible to collect a participant’s data for years or decades. Thus, we don’t know if the long-term effects of a drug are worse than the initial benefits.
Negative Study Results Aren’t Published
One of the significant challenges within the pharmaceutical industry is the prevalent practice of reporting only “positive” results from clinical trials. This selective disclosure not only paints an inaccurately optimistic picture of a drug’s efficacy and safety but also leads to a substantial waste of resources. Other pharmaceutical companies, unaware of the unsuccessful paths already explored, may invest billions of dollars into similar research avenues, only to arrive at the same dead ends. This cycle of redundant research not only inflates the cost and extends the timeline of drug development but also delays the availability of potentially life-saving treatments to the public. By not sharing comprehensive results—both positive and negative—the industry as a whole suffers from inefficient allocation of its research and development resources, stifling innovation and the progression of medical science.
Trials Often Aren’t Representative of Real Patients
Phase III clinical trials are often designed to exclude a vast majority of the population of interest. For example, only 14.5% of patients with depression meet the eligibility criteria for antidepressant trials. They exclude people who:
- take other medications
- use drugs or alcohol
- have milder forms of depression
- have co-occurring mental health conditions
So trial results only apply to a small, weird subset of patients instead of most of the people that will be actually be taking the drugs. This is why they rarely work as well in the real world as they do in trials.

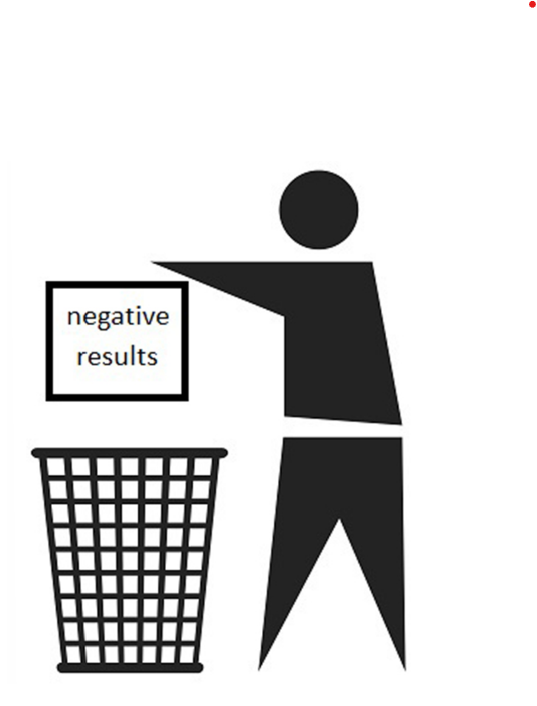
Trials Are Very Small
Trials are often very small and sometimes include only 20 people. This means lack the statistical power to detect the effects of a drug on rare side effects or specific subgroups of patients.
Trials are Short
Clinical trials can be as short as a few months. The short duration of clinical trials presents several significant problems that can impact the effectiveness and safety of new medications and treatments:
- Inadequate Safety Profiling: Short-term trials may not reveal all possible side effects, especially those that take time to develop. Some adverse effects, like organ damage or increased risk of chronic conditions, can only be observed after long-term exposure to a treatment.
- Lack of Long-Term Efficacy Data: Trials that run for a limited period may not provide enough evidence on the long-term benefits of a treatment. A drug might seem effective in the short term but could lose its efficacy over time or require increased doses, which could lead to additional side effects.
- Missing Late-Onset Side Effects: Certain side effects may emerge only after prolonged use of a medication. Short-term trials may conclude before these effects become apparent, leading to an incomplete understanding of the drug’s risk profile.
- Overlooking Cumulative Effects: Some drugs may have cumulative effects that only become significant over time. For example, a medication might gradually accumulate in the body and reach toxic levels after extended use, or it might cause gradual changes in the body that lead to health issues.
- Unidentified Drug Interactions: Patients often take multiple medications simultaneously, and interactions between drugs can develop over time. Short-term trials may not detect these interactions, which can lead to unforeseen complications when the drug is widely used.
- Neglecting Disease Progression: Many diseases, especially chronic conditions, progress over time. Short-term trials may not account for this progression and how it affects the efficacy or necessity of a treatment.
- Inability to Assess Quality of Life: The impact of a treatment on a patient’s quality of life is best assessed over a longer period, considering factors like daily functioning, mental health, and overall well-being.
- Poor Generalizability: Short-term trials often do not reflect real-world use, where patients may take a medication for years or even decades. This can lead to a misunderstanding of the treatment’s practical benefits and risks.
How Autonomous Clinical Research Could Solve These Problems
The creation of a super-intelligent FDAi AI agent, accessible to everyone, could revolutionize the landscape of clinical research by addressing several critical issues that currently plague the field. Here’s how this autonomous agent could transform clinical trials and research:
- Making Clinical Research More Economical: The astronomical figure of $2.6 billion needed to bring a drug to market, including the sunk cost of failed attempts, and the staggering $36k required per subject in Phase III clinical trials, underscores the financial burden of current research methodologies. An FDAi agent could significantly reduce these costs by automating the recruitment process, managing trial logistics, and streamlining data collection, making the development of new treatments more financially viable.
- Illuminating the Unknown Effects of Dietary Chemicals: With the long-term impacts of the vast majority of the 7,000+ synthetic or natural compounds in our food remaining largely unexplored due to limited research incentives, an FDAi agent could autonomously analyze data from real-world consumption and health outcomes. This would help identify potential health risks or benefits of these compounds, contributing to safer dietary practices and regulations.
- Reviving Interest in Off-Patent Treatments: The lack of financial incentive to explore new applications for off-patent drugs often leaves potentially beneficial treatments on the shelf. An FDAi agent could identify promising repurposing opportunities based on data analysis, supporting the investigation of off-patent drugs for new or rare diseases without the need for significant investment.
- Uncovering Long-Term Drug Effects: The current model, which finds it unfeasible to track long-term outcomes of drug use, results in a significant knowledge gap regarding a treatment’s prolonged impact. By continuously monitoring health data, an FDAi agent could provide insights into the long-term effects of medications, ensuring that their benefits genuinely outweigh any adverse effects.
- Ensuring Comprehensive Reporting: The prevalent practice of publishing only positive study results leads to redundant research efforts and hinders scientific progress. An FDAi agent could facilitate the sharing of all results, positive or negative, ensuring a comprehensive understanding of each treatment’s efficacy and safety.
- Widening the Scope of Trial Participation: The restrictive nature of current trial eligibility criteria limits the diversity and representativeness of study participants. By leveraging an FDAi agent for patient recruitment, trials could include a broader cross-section of the population, ensuring findings are applicable to a wider group of patients.
- Addressing the Limitations of Trial Size and Duration: The often small and short-term nature of clinical trials limits their ability to detect rare side effects, assess long-term efficacy, and understand cumulative and late-onset effects. An FDAi agent could facilitate larger, longer, and more adaptive trials, improving the quality and reliability of research outcomes.
By addressing these issues, the introduction of a personal AI doctor and clinical research agent for everyone could not only make clinical research faster, cheaper, and more precise but also ensure that the development and approval of new treatments are based on comprehensive, representative, and long-term data. This represents a significant leap towards personalized medicine, safer treatments, and a broader understanding of both the benefits and risks of medical interventions.
The FDAi Act proposes the creation of a super-intelligent AI assistant, freely available to the public, that could make by:
- Automating the Clinical Trial Process: An autonomous FDAi agent could automate patient recruitment and trial management. The FDAi would proactively identify individuals for clinical trial participation, facilitate effortless enrollment, manage medication shipments, schedule lab tests, and continuously collect data on treatment efficacy and side effects.
- Expanding Trial Participation: With a far lower barrier to entry, we could vastly increase the number of people participating in clinical trials, including those traditionally excluded. This would provide a more accurate representation of how drugs work across diverse populations.
- Enhancing Precision Medicine: By analyzing a wealth of data, the FDAi agent could tailor treatments to individual genetic profiles, health histories, and lifestyle factors, bringing us closer to the dream of the personalized, precision medicine of the future.
Implementation of the FDAi Agent
The FDAi agent would use causal inference to differentiate between mere associations and actual causes of health outcomes. It would employ black box optimization to refine its predictions and recommendations continuously. By applying control systems theory, the agent would also maintain the balance of inputs (like diet, medication, and lifestyle factors) to optimize health outcomes.
How FDAi Agents Would Make Research More Accurate
There are 2 billion people suffering from chronic illness that do not have access to an adequate treatment. Yet, less than 1% of people can participate.
If they all had a personal FDAi agent, it would be possible to vastly expand the pool of trial participants to include almost everyone who could benefit from a treatment.
Instead of patients having to find trials, the trial can find the patients based on their actual up-to-date health data. This means trials could enroll far more diverse, representative participants.
The FDAi agent would also enable decentralized trials so anyone can participate from home. This further removes barriers to entry and makes clinical research more accessible and inclusive.
How it Works
By incorporating the FDAi personal AI agent into the clinical trial framework, we move toward a much more efficient and economical model of conducting research. Automation in patient recruitment and data collection would make trials cheaper and lowering barriers and democratizing access to innovative medical treatments.
1. Automated Patient Recruitment
One of the most challenging and expensive aspects of clinical trials is identifying and recruiting suitable candidates. The FDAi personal AI agent could automate away this mess! By tapping into extensive databases and electronic health records, the FDAi could identify individuals who meet the specific criteria for a given trial lickety split!
Instead of relying humans to do boring manual screening, the FDAi agent can send targeted invitations to those already pre-qualified based on their medical histories. This method reduces recruitment time and costs associated with outreach and pre-screening.
2. Automating Data Collection
Another critical aspect where the FDAi agent can drastically reduce costs is in data collection. Traditionally, data is gathered through periodic visits to clinical sites, which involves significant expenses and participant stipends. The FDAi agent can continuously collect real-time data remotely through phone calls, wearables, mobile applications or whatever you want!
Patients can have their vitals monitored, medication adherence tracked, and side effects recorded without the need for frequent clinic visits. This not only reduces the cost burden on the sponsors of the trials but also improves patient compliance and retention by minimizing the inconvenience to participants.
3. Automating Data Management
Data collected during clinical trials are vast and complex, often leading to high costs in data management. The FDAi agent, with its superior robot brain, could automate data entry, integration, and analysis, reducing the need for manual data handling and the potential for human error. This would decrease administrative overheads and the cost of people needed to manage the data.
This is Urgent!
With less than 1% of people with chronic diseases participating in clinical trials, many potentially life-saving treatments are not being explored. The FDAi agent could find the most promising new treatments for individuals, reducing the time and suffering associated with prolonged illness.
Supporting the FDAi Act means a future where every patient has access to cutting-edge, personalized medical treatment. It’s about transforming clinical research from a slow, expensive, and imprecise process into a fast, inclusive, and accurate one. By supporting this act, we take a significant step towards minimizing the suffering caused by serious diseases and optimizing health outcomes for all.